2Department of Radiation Oncology, Max Super Speciality Hospital, Shalimar Bagh, Delhi-India
3Department of Physics, University School of Automation and Robotics, Guru Gobind Singh Indraprastha University, East Campus, Delhi-India
4Radiation Oncology Centre, Army Hospital Research and Referral, Delhi Cantonment, New Delhi-India
5Department of Radiation Oncology, Ranchi Cancer Hospital and Research Centre (Tata Trusts), Ranchi, Jharkand-India DOI : 10.5505/tjo.2023.4003
Summary
OBJECTIVEThe purpose was to observe the impact of Equivalent dose of 2Gy (EQD2) for different planning technique combined with intracavitary brachytherapy (ICBT) for cervix patients and to manage the organ at risks (OARs) doses in external beam radiation therapy (EBRT) and brachytherapy to respect the EQD2 tolerances.
METHODS
Retrospectively, 15 patients of federation of gynecologists and obstetricians Stage IB-IVA, received a
dose of 45Gy in 25 fractions with a simultaneous integrated boost of 55Gy in 25 fractions to the nodes
with EBRT followed by three applications of ICBT of a dose of 8Gy, were selected. Intensity modulated
radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT) plans were created for each patient
with a single iso-center with 6MV photon energy. EQD2 of D2cc, D1cc, and D0.1cc of bladder and
rectum were compared for IMRT followed by ICBT and VMAT followed by ICBT.
RESULTS
The IMRT and VMAT plans were comparable in terms of target coverage and OARs sparing. The conformity
index and homogeneity index were comparable for both IMRT and VMAT with p=0.007. In
VMAT and ICBT plan the EQD2 of D2cc, D1cc, and D0.1cc for bladder were reduced 0.66%, 0.41%, and
0.41%, respectively, from IMRT and ICBT plan.
CONCLUSION
We recommend following VMAT and ICBT over IMRT and ICBT due to advantages of VMAT over
IMRT and try to keep doses of OARs as low as possible in both EBRT and BT separately.
Introduction
Globally, cervical cancer was reported as the fourth most common cancer type. In India, the cervical cancer is second most common cancer among women and in 2020, more than six lac cases were reported.[1] In cervical cancer, the federation of gynecologists and obstetricians staging method is followed to decide the mode of treatment.[2] According American brachytherapy society, external beam radiation therapy (EBRT) followed by brachytherapy decreases the recurrence rate and increases the survival rate in cervical cancer patients of stage IB2-IVA.[3] The types of brachytherapy application such as intracavitary or interstitial are decided on the tumor response of tumor and primary disease extension.Equieffective or equivalent doses were defined as absorbed doses that, when delivered under specified but different conditions produce the same probability of a specific radiation effect or endpoint. Equivalent dose of 2Gy (EQD2) implies that when two or more radiation schedules were compared, the reference treatment was delivered by 2Gy per fraction.[4] Linear quadratic model formalism and EQD2 allow comparison of the predicted effects of a particular brachytherapy schedule with other brachytherapy and external beam schedules, with regard to both tumor control and normal tissue effects. This formalism can be safely applied within a range of doses per fraction from 0.5Gy to 10Gy.[4] It might, however, potentially overestimate the effects at higher doses per fraction.[4] Therefore, international commission on radiation units and measurements (ICRU)-89 and Groupe Européen de Curiethérapie and European Society for Radiotherapy and Oncology reports recommended the use of the equieffective formalism, particularly EQD2, for the addition of absorbed doses to report doses for planning aims, prescriptions, and doses delivered.[4,5]
Intensity modulated radiotherapy (IMRT) involves the basic principle of irradiation of target from various directions with radiation beams that are optimized by inverse planning to provide a high dose to the tumor site and an acceptably low dose to healthy normal tissues using treatment planning system (TPS).[6] The major limitation of IMRT is a large number of MU's, time consuming. Volumetric modulated arc therapy (VMAT) is the advanced IMRT technique, which has gained popularity as a means of overcoming these restriction with VMAT, better conformal dose distribution could be achieved.[7]
Prior research was conducted for intracavitary brachytherapy (ICBT), on the effects of various fractionation systems on EQD2 or the effects of IMRT and 3DCRT planning strategies on EQD2.[8-10] As a result, this study decided to conduct an analogous study using modern planning techniques. The current study's objective was (1) to evaluate the impact of IMRT and VMAT on the cumulative EQD2 of both EBRT followed by brachytherapy for organ at risk (OARs) like bladder and rectum. (2) Whether the planning strategies could be altered the dosimetric parameter significantly. (3) The range of doses could be tried to achieve for individually in EBRT and ICBT for the bladder and rectum to respect the OARs and target EQD2.
Methods
Patient SelectionBetween 2018 and 2022, 26 patients were retrospectively selected for this pilot study. The patients had uterine cervix cancer with Stage IB-IVA and were scheduled for radical radiotherapy with external beam radiotherapy and three-fraction high dose ICBT, were included. The patient who received palliative radiation or had extended treatment field, that is, length more than 32 cm, because of jaw size limitations and VMAT plans could not be created with single isocenter or did not received three-fraction ICBT, were excluded from the study. On the basis of the mentioned criteria, we had eliminated five patients from our study. Therefore, total 21 patients were selected for the study. The average planning target volume (PTV) length superior to inferior for patients was 21.54±3.68 cm.
SIMULATION
EBRT
Patients were simulated under bowel and bladder protocol.
During the bowel protocol, patients were given
8 mL of contrast diluted in 500 mL of water orally. After
1 h, a check scan was performed to ensure proper
rectal filling. If the rectum was found to be more than
3 cm dilated at any level, a proctolysis enema was
administered. Using hands above the head or on the
chest, a 4-clamp thermoplastic mask was used made
to immobilize the patient from the chest to the middle
of the thigh. For bladder protocol, the patients were
given 500 mL of water orally instructed to wait 20-30
min, or until they felt their bladders were full. A rectal
tube with the length of 3-4 cm and 2 mL of contrast
diluted in 10 mL of normal saline was inserted in the rectum. Radio-opaque marker was placed over the distal
most end of the disease for upfront radiotherapy or
on vaginal volt for post-operated cases and introitus.
Contrast-enhanced computed tomography (CT) data
were obtained from T12 to mid-thigh with 3 mm thick
contiguous slices with CT simulator (Discovery RTCT,
General Electric Healthcare, USA). The patient was
evaluated by a clinician during the final week of EBRT
to determine if ICBT or interstitial brachytherapy was
appropriate.
Brachytherapy
A day before the procedure enema was given to the patient
for bowel preparation. Before starting the procedure
in the morning, mexaprost was given to patients
for cervix dilation as it helped in the easy insertion of
the applicator. During application, the rectal tube with
length of 3-4 cm was inserted and during simulation
10 mL (1 mL contrast in 9 mL water) of diluted contrast
was injected into the bladder through Foley's catheter
and same amount of contrast was inserted to rectum
for better delineation. Under ultrasound guidance,
the appropriate/suitable applicators were inserted by
the radiation oncologist. All three fractions of brachytherapy
treatment were performed using Fletcher-Suite
Delclos-Style applicator-flexible geometry (Varian,
AL1303001) was used. CT data of 2.5 mm slice thickness
were acquired from S1 level to vulva level with the
same CT simulator.
CONTOURING
EBRT
Contouring of clinical target volume (CTV), PTV
was done as per EMBRACE II protocol.[11] Gross
Disease visualized on MRI imaging (magnetic resonance
imaging) and PET-CT was contoured as gross
tumor volume. CTV was contoured 2 cm distal end
of the vagina including the vaginal wall, cervix, uterus,
fallopian tube, and ovaries. The anterior border
of CTV was limited to include 5 mm of the posterior
surface of the bladder, while posterior contour included
anterior wall of the rectum. The lateral extend
of the CTV contour was kept at the lateral pelvic wall.
The superior end of the CTV lymph node in case of
node negative disease was kept at the bifurcation of
common iliac vessels. The OARs contoured as per
the RTOG atlas were rectum, bladder, sigmoid colon,
large bowel, small bowel, bilateral femur head, bowel
bag, bilateral kidneys, and liver. The bowel bag was
contoured a minimum 2 cm superior of PTV.
Brachytherapy
For brachytherapy, the bladder was contoured as the
whole organ inferiorly from the base and superiorly to
the dome. The rectum was delineated as 1 cm from the
anus to the recto-sigmoid transition through the entire
thickness of the organ wall. It ends superiorly before
the rectum loses its round shape in the axial plane.
Other organs such as sigmoid and bowel were also contoured.
Sigmoid was contoured from the AnoRectum
junction to descending colon laterally.
DOSE PRESCRIPTION
EBRT
External beam radiation was delivered with a dose 45Gy
in 25 fractions in 5 weeks for the pelvis with simultaneous
integrated boost (SIB) boost to lymph nodes at a
dose of 55Gy in 25 fractions. OARs dose constraints
were kept as per EMBRACE II protocol.[11]
Brachytherapy
Within 1 week (4-7 days post EBRT completion) following
the completion of external beam radiation,
brachytherapy was started. A dose of 24Gy in three
fractions was delivered with each fraction scheduled at
an interval of 4-7 days such that the whole treatment
complete within 8 weeks.
PLANNING
EBRT
All plans were generated with single isocenter irrespective
of treatment field length and 6MV energy in Eclipse
TPS (version 13.7; Varian Medical Systems, Inc., Palo
Alto, CA, USA) for linear accelerator (True Beam STX;
Varian Medical Systems, Inc., Palo Alto, CA, USA).
In the VMAT plans, for patients with treatment field length <22 cm, full two coplanar arc were used and more than 22 cm, full three coplanar arcs were used due to Y jaw limitation in True Beam LINAC with HD MLC. For fields >22 cm PTV length, X jaw was opened asymmetrically with collimeter 90° for two arc fields. The Y jaw was opened according to PTV width. The remaining arc field was placed with symmetric X jaw, collimator angle between 355° and 5°, and Y jaw opened 22 cm. The plans were optimized with photon optimizer algorithm. The isocenter was placed nearly to the center of the PTV.
In IMRT plans, eight fields for all patients with gantry angles 40°, 80°, 120°, 160°, 200°, 240°, 280° and 320°. The planning aim was to 95% volume of the PTV should be covered at least 95% of prescribed dose and minimal dose to OARs. The isodose levels of both the plans are shown below in Figure 1.
Brachytherapy
The brachytherapy planning was done in Brachy Vision
Planning System (version 13.7; Varian Medical
Systems, Inc., Palo Alto, CA, USA). The colpostat
tandem (left and right) and intrauterine tandem were
reconstructed manually. A 0.6 cm offset was specified
for both intrauterine and colposate tandems. The average
source loading in colpostat was 2 cm and in uterine
tandem was 5 cm. The plans were normalized at point
A (2 cm superior from the surface of the ovoids and 2
cm lateral from central uterine tube). The prescribed
dose for each application was 8Gy. The isodose distribution
of ICBT plan is shown in Figure 2 below.
Dosimetric Details
Dose volume histogram was used to evaluated the PTV
and OAR's dose. In EBRT, the plan quality was analyzed using following parameters: D95%, D98%, D2%, V95%,
V105%, homogeneity index (HI), and conformity index
(CI) where D95%, D98%, and D2% are dose to 95%, 98%,
and 2%, of the volume, respectively, and V95% and V105%
are defined as volume covered with 95% and 105% of
the prescribed dose, respectively.
The HI was calculated using following formula.[12]
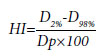
Where D2% and D98% are dose to 2% and 98% of the volume and Dp is the prescribed dose.
The CI was calculated using following formula.[13]
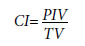
PIV: Volume enclosed by the prescribed isodose volume; TV: volume of the target volume
For OAR's, D2cc, D1cc, and D0.1cc of the bladder and rectum were evaluated for both EBRT and Brachytherapy plans.
For EQD2 calculation for EBRT and brachytherapy planned dose, the following formula was used.[14]
EQD2=Nd (1+gd/(α/β))/(1+2/(α/β))
Where N, d, and g represent the number of fractions, dose per fraction, and an incomplete repair function respectively. g=1 for high dose rate brachytherapy. α/β=10 for tumor and α/β=3 for OARs.
Statistical Analysis
The Wilcoxin signed ranked test was performed to
analyze the difference in dosimetric parameters and
p≤0.05 was considered statistically significant.
Results
From the Table 1, the dosimetric parameters of PTV D95%, D98%, D2%, and V95% were comparable in both IMRT and VMAT plans except V105% which was comparatively higher in IMRT plans than VMAT plans. Therefore, the IMRT and VMAT plans were comparable in terms of CI and HI. The MU and treatment time of VMAT plan was significantly less than IMRT plans making it superior to IMRT plans. On an average, the VMAT MU's and treatment time both were 0.75%±0.05% lesser than IMRT MU'S and treatment time.In Table 2, The dosimetric parameters D2cc, D0.1cc, and D1cc evaluated for bladder and rectum were statistically comparable for both IMRT and VMAT plans. On an average, the D2cc of bladder is 106.04%±5.11% of the prescribed dose (prescribed dose was 45Gy to PTV) in VMAT cases in comparison to 106.80%±5.59% (prescribed dose was 45Gy to PTV) IMRT cases. The range of variation for D2cc of bladder for VMAT cases was 91.3% to 110.44% of the prescribed dose to PTV and for IMRT cases, it varies from 93.12% to 110.62% of the prescribed dose to PTV.
Similarly for rectum, the average D2cc was 102.1%±2.4% of the prescribed dose (prescribed dose was 45Gy to PTV) for VMAT cases and 102.2%±1.81% (prescribed dose was 45Gy to PTV) for IMRT cases. The range of variation of D2cc for rectum in IMRT cases was 99.2-105.2% of the prescribed dose to PTV and in VMAT the range was from 98.4% TO 108.1% of the prescribed dose to PTV.
The D2cc for bladder was higher than rectum for both the IMRT and VMAT techniques.
According to Table 3, there is no significant difference in EQD2 doses of D2cc, D0.01cc, and D1cc of both bladder and rectum for IMRT and ICBT and VMAT and ICBT plans. The results were comparable. The average percentage variation in EQD2 of D2cc parameter between IMRT and VMAT was 0.53%±0.99% for bladder and 0.004%±1.16% for rectum. The variation was <1%.
Table 3 Combined average EQD2 of D2cc, D0.1cc, D1cc IMRT+ICBT and VMAT+ICBT plans for 21 patients
According to Table 4, BED of D2cc parameter of bladder and rectum for both IMRT and ICBT plans and VMAT and ICBT plans was comparable. The average percentage variation in BED of D2cc parameter between IMRT and VMAT was 0.53%±0.99% for bladder and 0.004%±1.16% for rectum. The variation was <1%.
Table 4 Combined average BED of D2cc, D0.1cc, D1cc IMRT+ICBT and VMAT+ICBT plans for 21 patients
In Figure 3, The EQD2 of D2cc parameter of bladder ranges from 111.56Gy to 63.16Gy in both IMRT and ICBT and VMAT and ICBT cases with five patients being outlier having EQD2 greater than 90Gy. The range of EQD2 of D0.1cc and D1cc parameter of bladder for both IMRT and ICBT and VMAT and ICBT ranges from 78.53Gy to 156Gy and 66.24Gy to 126.95Gy, respectively.
In Figure 4, Graphically, the EQD2 of D2cc of rectum was comparable for both the plans IMRT+ICBT and VMAT+ICBT.
The EQD2 of D2cc parameter of rectum ranges from 82.66Gy to 50.05Gy in both IMRT and ICBT and VMAT and ICBT cases with five patients being outlier having EQD2 greater than 90Gy. The range of EQD2 of D0.1cc and D1cc parameter of bladder for both IMRT and ICBT and VMAT and ICBT ranges from 53.95Gy to 107.96Gy and 50.88Gy to 88.71Gy, respectively.
Discussion
According to the NCI alert 1999,[14] standard treatment care for cancer of cervix was concurrent chemoradiation followed by brachytherapy.[14] Radiation therapy includes radiation to the pelvis with or without the inclusion of the para-aortic Lymph node region depending on lymph node status.[11] In cervix cases, the treatment protocol was EBRT followed by brachytherapy as a boost.[15] IMRT had proven to be more conformal in terms of dose distribution in comparison to conventional treatment in cancer cervix in terms of organ sparing and dose coverage.[16,17] VMAT technique was another method to deliver IMRT with certain benefits over IMRT.[18] In our study, the IMRT and VMAT plans were comparable dosimetrically The average MU's delivered in VMAT plans were 636.69±135.55 in comparison to 2683.65±952.71 in IMRT. The treatment time for VMAT plans was 1.06 min±0.23 min and for IMRT 4.47 min±1.55 min. The difference is quite appreciable logistically. Moreover, VMAT plans had comparatively lower rectum and bladder doses than IMRT plans. Bai et al.[19] stated that in comparison to IMRT plan, VMAT plans were more protective for rectum and had also significantly reduced MU's as well. Zhai et al.[20] concluded that there was no significant dosimetric benefit of VMAT over IMRT except fewer MU's and faster treatment. Sharma et al.[21] also stated the same, that treatment delivery efficiency was higher with VMAT plans in comparison to IMRT plans with equivalent target coverage and OARs doses.In retrospective study of 21 patients, observed variation between EQD2 of D2cc of IMRT and ICBT and VMAT and ICBT was in the range of -0.61 Gy to 2.43Gy for bladder and -2.74 Gy to 0.9Gy for the rectum. The range of variation for OARs for both the combined techniques, that is, IMRT and ICBT and VMAT and ICBT was small and comparable. Therefore, on the basis of our findings, we recommend to opt for VMAT and ICBT over IMRT and ICBT.
External beam radiation was delivered with a dose 45Gy in 25 fractions in 5 weeks for the pelvis with SIB boost to lymph nodes at a dose of 55Gy in 25 fractions. A dose of 24Gy in three fractions was delivered in brachytherapy.[9] Therefore, combined EQD2 of EBRT and BT to the target is optimal, that is, 81Gy. As per ICRU89, the EQD2 of the target should be in the range of 80Gy to 90Gy. Tanderup et al.[22] reported that a better local control rate was observed with EQD2 of target ≥85Gy. Dimopoulos et al.[23] also reported that patients who received EQD2 ≥87Gy had better local control and lower chances of recurrences. Mazeron et al.[24] reported that EQD2 of D2cc more than 75Gy in the rectum, chances of Grade 3 and high rectal complications is increased. Georg et al.[25] reported in their study that there is an increased probability of Grade 3 rectal toxicities for a dose greater than 76Gy and 88Gy for D2cc and D0.1cc of the rectum, respectively. In a retrospective study by Manir et al.[26] on the correlation between rectal toxicity and dose, it was recommended to restrict the EQD2 dose between 64Gy to 69Gy and 75Gy to 81Gy for D2cc and D0.1cc respectively of the rectum to avoid grade 3 proctitis. Romano et al.[27] stated that genitourinary toxicity Grade 3+ rate increases from 3.6% to 5.6% as the EQD2 of D2cc the bladder increases from 80Gy to 90Gy. Therefore, we should try to aim an EQD2 of D2cc < 80Gy for bladder although the threshold is 90Gy.
The combined EQD2 of brachytherapy and EBRT limits for OARs is as follows: 90Gy for bladder and 75Gy for rectum.[3,28,29] It is necessary to keep the EQD2 of OARs as low as possible to reduce toxicity without compromising the EQD2 of the target. We concluded that to respect the cumulative dose for bladder and rectum, EBRT D2cc for bladder and rectum should be <107% of the prescribed dose and BT D2cc for the bladder should be in the range of 75-88% of the prescribed dose (6Gy-7.04Gy of the prescribed dose 8Gy) and for rectum, it should be between 53%-68% of the prescribed dose (4.24Gy-5.44Gy of the prescribed 8Gy). Therefore, the combined EQD2 should be in the range of 77Gy-90Gy for the bladder and 65.5Gy to 74.7Gy for the rectum. For bladder, D0.1cc should be in range of 90-100% of the prescribed dose (7.2Gy-8Gy of the prescribed dose). For rectum, D0.1cc should be maintained in within 68.75-75% of the prescribed dose (5.5Gy-6Gy of the prescribed dose). The D1cc is not a strong predictor for rectal as well as bladder toxicity; therefore, we had just recorded it.[25] Moreover, there is very limited clinical data to justify the significance of D1cc.
Limited patient data were one of the limitations of our study. We had considered the ICBT application of brachytherapy for our study. A similar study can be conducted in interstitial and vaginal brachytherapy cases in future. We had limited our study to bladder and rectum only which can be extended to other OARs like sigmoid and bowel as well in the future. It is an institutional study which was conducted with an aim to encourage the use of VMAT planning technique as a practice instead of IMRT and to be very cautious about the doses of OARs in EBRT as well BT during planning so that we need not to compromise on BT dose to respect the EQD2 tolerances of OARs.
Conclusion
Although the EQD2 of combined IMRT and ICBT and VMAT and ICBT plans were comparable, still we recommend adapting VMAT and ICBT over IMRT and ICBT due to added advantage of lesser MU's and treatment time with comparable target coverage and OARs sparing in VMAT over IMRT. Moreover, we should maintain the doses of both bladder and rectum such that it should not exceed 107% of the prescribed dose in EBRT cases and D2cc of bladder and rectum should be 75-88% and 53-68% respectively of the prescribed dose in brachytherapy to respect the combined EQD2 tolerances of OARs.Acknowledgement: I would like to thank Mr. Pawan Kumar Singh for his constant support and guidance.
Peer-review: Externally peer-reviewed.
Conflict of Interest: All authors declared no conflict of interest.
Financial Support: None declared.
Authorship contributions: Concept - N.M., D.T., N.K., D.K.; Design - N.M., M.K.S., D.K., N.K.; Supervision - N.M., D.T., N.K., D.K., R.K.; Materials - R.K., D.K., M.K.S., N.M., D.T., N.K.; Data collection and/or processing - N.M., N.K., M.K.S., D.T.; Data analysis and/or interpretation - N.M., N.K., D.T., R.K., D.K.; Literature search - N.M., M.K.S., N.K., D.K.; Writing - N.M., N.K., D.K., M.K.S.; Critical review - N.K., D.K., M.K.S., R.K., D.T.
References
1) International Agency for Research on Cancer. India
Globocan 2020. Available at: https://gco.iarc.fr/today/
data/factsheets/populations/356-india-fact-sheets.
pdf. Accessed Apr 20, 2021.
2) Salib MY, Russell JHB, Stewart VR, Sudderuddin SA,
Barwick TD, Rockall AG, et al. 2018 FIGO staging
classification for cervical cancer: added benefits of
imaging. Radiographics 2020;40(6):1807-22.
3) Viswanathan AN, Thomadsen B; American
Brachytherapy Society Cervical Cancer Recommendations
Committee; American Brachytherapy Society.
American Brachytherapy Society consensus guidelines
for locally advanced carcinoma of the cervix. Part
I: general principles. Brachytherapy 2012;11(1):33-46.
4) Pötter R, Kirisits C, Erickson B, Haie-Meder C, Van
Limbergen E, Lindegaard J. C, et al. ICRU report 89:
Prescribing, recording and reporting brachytherapy for
cancer of the cervix. Journal of the ICRU 2013;13(1-2).
Available at: https://conferences.iaea.org/event/142/
contributions/4622/attachments/2964/3553/ICRU_
Report_89.pdf. Accessed Apr 19, 2023.
5) Pötter R, Haie-Meder C, Van Limbergen E, Barillot I, De
Brabandere M, Dimopoulos J, et al; GEC ESTRO Working
Group. Recommendations from gynaecological
(GYN) GEC ESTRO working group (II): concepts and
terms in 3D image-based treatment planning in cervix
cancer brachytherapy-3D dose volume parameters and
aspects of 3D image-based anatomy, radiation physics,
radiobiology. Radiother Oncol 2006;78(1):67-77.
6) Taylor A, Powell ME. Intensity-modulated radiotherapy--
what is it? Cancer Imaging 2004;4(2):68-73.
7) Guy JB, Falk AT, Auberdiac P, Cartier L, Vallard A,
Ollier E, et al. Dosimetric study of volumetric arc
modulation with RapidArc and intensity-modulated
radiotherapy in patients with cervical cancer and comparison
with 3-dimensional conformal technique for
definitive radiotherapy in patients with cervical cancer.
Med Dosim 2016;41(1):9-14.
8) Christensen EN, Yu HZ, Klopp AH, Tsai JC, Lawyer
AA, Court LE, et al. Variable impact of intracavitary
brachytherapy fractionation schedule on biologically
effective dose to organs at risk in patients with cervical
cancer. Brachytherapy 2014;13(3):240-9.
9) Rao BS, Das P, Subramanian BV, Jena A, Rashmi P,
Konakalla VLA, et al. A comparative analysis of two
different dose fractionation regimens of high dose rate
intracavitary brachytherapy in treatment of carcinoma
of uterine cervix: a prospective randomized study. J
Clin Diagn Res 2017;11(4):XC01-XC05.
10) Dröge LH, von Sivers FF, Schirmer MA, Wolff HA.
Conventional 3D conformal radiotherapy and volumetric
modulated arc therapy for cervical cancer:
Comparison of clinical results with special consideration of the influence of patient- and treatment-related
parameters. Strahlenther Onkol 2021;197(6):520-7.
11) Pötter R, Tanderup K, Kirisits C, de Leeuw A, Kirchheiner
K, Nout R, et al; EMBRACE Collaborative
Group. The EMBRACE II study: The outcome and
prospect of two decades of evolution within the GECESTRO
GYN working group and the EMBRACE studies.
Clin Transl Radiat Oncol 2018;9:48-60.
12) Kataria T, Sharma K, Subramani V, Karrthick KP, Bisht
SS. Homogeneity Index: An objective tool for assessment
of conformal radiation treatments. J Med Phys
2012;37(4):207-13.
13) Petrova D, Smickovska S, Lazarevska E. Conformity
index and homogeneity index of the postoperative
whole breast radiotherapy. Open Access Maced J Med
Sci 2017;5(6):736-9.
14) Josefson D. Adding chemotherapy improves survival
in cervical cancer. BMJ 1999;318(7184):623.
15) Ager BJ, Torgeson A, Francis SR, Burt LM, Gaffney
DK, Cannon DM. Impact of brachytherapy boost and
dose-escalated external beam radiotherapy in margin
positive cervical cancer treated with chemotherapy
and radiation. Am J Clin Oncol 2020;43(1):35-42.
16) Mundt AJ, Lujan AE, Rotmensch J, Waggoner SE, Yamada
SD, Fleming G, et al. Intensity-modulated whole
pelvic radiotherapy in women with gynecologic malignancies.
Int J Radiat Oncol Biol Phys 2002;52(5):1330-7.
17) Portelance L, Chao KS, Grigsby PW, Bennet H, Low D.
Intensity-modulated radiation therapy (IMRT) reduces
small bowel, rectum, and bladder doses in patients with
cervical cancer receiving pelvic and para-aortic irradiation.
Int J Radiat Oncol Biol Phys 2001;51(1):261-6.
18) Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A.
Volumetric modulated arc therapy: a review of current
literature and clinical use in practice. Br J Radiol
2011;84(1007):967-96.
19) Bai W, Kou C, Yu W, Li Y, Hua W, Yu L, et al. Dosimetric
comparison of volumetric-modulated arc therapy
and intensity-modulated radiation therapy in patients
with cervical cancer: a meta-analysis. Onco Targets
Ther 2018;11:7179-86.
20) Zhai DY, Yin Y, Gong GZ, Liu TH, Chen JH, Ma CS,
et al. RapidArc radiotherapy for whole pelvic lymph
node in cervical cancer with 6 and 15 MV: a treatment
planning comparison with fixed field IMRT. J Radiat
Res 2013;54(1):166-73.
21) Sharma MK, Mitra S, Saxena U, Bhushan M, Shrivastava
H, Simson DK, et al. Is volumetric modulated arc
therapy (RapidArc) better than intensity modulated radiotherapy
for gynecological malignancies? A dosimetric
comparison. J Cancer Res Ther 2014;10(4):883-8.
22) Tanderup K, Fokdal LU, Sturdza A, Haie-Meder C,
Mazeron R, van Limbergen E, et al. Effect of tumor
dose, volume and overall treatment time on local control
after radiochemotherapy including MRI guided
brachytherapy of locally advanced cervical cancer. Radiother
Oncol 2016;120(3):441-6.
23) Dimopoulos JC, Lang S, Kirisits C, Fidarova EF, Berger
D, Georg P, et al. Dose-volume histogram parameters
and local tumor control in magnetic resonance imageguided
cervical cancer brachytherapy. Int J Radiat Oncol
Biol Phys 2009;75(1):56-63.
24) Mazeron R, Fokdal LU, Kirchheiner K, Georg P, Jastaniyah
N, Segedin B, et al; EMBRACE collaborative
group. Dose-volume effect relationships for late rectal
morbidity in patients treated with chemoradiation
and MRI-guided adaptive brachytherapy for locally
advanced cervical cancer: Results from the prospective
multicenter EMBRACE study. Radiother Oncol
2016;120(3):412-9.
25) Georg P, Lang S, Dimopoulos JC, Dörr W, Sturdza AE,
Berger D, et al. Dose-volume histogram parameters
and late side effects in magnetic resonance imageguided
adaptive cervical cancer brachytherapy. Int J
Radiat Oncol Biol Phys 2011;79(2):356-62.
26) Manir KS, Patra NB, Mukherjee A, Basu S, Sarkar SK,
Pal JK, et al. A retrospective study of correlation between
reported dose volume parameters for urinary
bladder, rectum & sigmoid colon with clinical outcome
in high dose rate brachytherapy of carcinoma
cervix. IOSR J Dental Med Sci 2016;15(7):14-23.
27) Romano KD, Hill C, Trifiletti DM, Peach MS, Horton
BJ, Shah N, et al. High dose-rate tandem and ovoid
brachytherapy in cervical cancer: dosimetric predictors
of adverse events. Radiat Oncol 2018;13(1):129.





