2Department of Radiotherapy, Bezmialem Vakif University, İstanbul-Türkiye DOI : 10.5505/tjo.2022.3722
Summary
OBJECTIVEIn total body irradiations (TBI), it becomes hard getting homogenous dose distribution due to inhomogeneous body shape and tissue density variations. The main goal of the radiotherapy is saving critic organs while achieving a homogeneous dose distribution at target volume. Tomotherapy is a new and one of the most effective technologies used for TBIs. In this study, it was aimed to investigate TBI planned target volume coverage and organ doses using helical tomotherapy.
METHODS
To simulate rando phantom geometrically, arms and legs created by rice are added to rando phantom
and were immobilized by a vacuum bed. The computer tomography images of the lower and upper
region of the phantom with a slice thickness of 3 mm were acquired. Thermoluminescent dosimeters
(TLDs) were placed entrance-exit, midpoint doses of lens, lungs, and kidneys onto a rando phantom.
The treatment plans were prepared and irradiated with helical tomotherapy technique. Results of the
measurements and treatment planning system (TPS) doses were compared. All procedures were repeated
3 times and averaged. TPS and TLD doses were evaluated by Wilcoxon test.
RESULTS
In the plans prepared with the TPS, a homogeneous dose distribution was obtained with a homogeneity
index of 0.16 for the upper body and 0.04 for the lower body. When the calculated critical organ doses
were compared with the measured organ doses, there was no statistically significant difference between
them.
CONCLUSION
It was seen that saving critical organs and achieving desired dose distribution are possible without blocks
in tomotherapy. Doses calculated from TPS and measured by TLD matched.
Introduction
Total body irradiation (TBI) is a type of radiotherapy that is applied with chemotherapy as a preparative regime for bone marrow transplantation and stem cell transplantation. Main purposes of the TBI are to eliminate malignant cells, to prevent graft rejections, and to become ready for transplantation.[1,2]In TBI s, it becomes hard getting homogenous dose distribution due to inhomogeneous body shape and tissue density variations. The main goal of the radiotherapy is saving critical organs while achieving a homogeneous dose distribution in the target volume.
There has been a wide range of TBI treatment techniques from the past to now. While classical TBI treatments were performed with Co-60 and long source skin distance (SSD) Linac in the past, today treatments are applied using advanced technologies such as volumetric modulated arc therapy (VMAT) and tomotherapy. When old technologies are preferred, external shielding blocks or compensators are used and for long SSD Linac treatments, big treatment room size is needed in the clinic. Treatment room size, patient comfort, and treatment times are important factors to feel the need for developing technologies for TBI treatments. The big treatment room size, time taking factors like shielding blocks, and compensators are not needed in the new technologies. Treatment time is also another important advantage of tomotherapy and VMAT. Recently, the most preferred and effective two technologies used in TBI treatments which are as follows: Tomotherapy and VMAT. Tomotherapy is a new and one of the most effective technologies used for TBIs. It is a 6 MV machine that is a combination of computed tomography (CT) and intensity modulated radiation therapy. Since it gives MVCT images before each treatment, appropriate patient localization, and beam delivery can be achieved in tomotherapy.[3,4] During the tomotherapy treatment gantry, couch and multileaf collimators perform interrelatedly and helical beam delivery occurs. Without the need for external shielding blocks, appliers can save critical organs and irradiate 40×160 cm2 size of targets at 1 time. Latter is the most important advantage of tomotherapy for TBI treatments.[5,6]
In TBI treatments, the most common dose fraction schedule is12 to 15 Gy given in 8 to 12 fractions over 4 days, with 2 to 3 treatments daily.[7-9] Since patients get high doses in single or fractionated TBI treatments, some late effects can be seen such as endocrine, metabolic, renal, eye, and neurocognitive abnormalities. [10] Therefore, it is necessary to be sure correctness of the given treatment. To ensure that, in vivo dosimetry is needed. ln vivo dosimetry is strongly recommended to determine dose homogeneity, to check patient position, and to reproduce treatment.[11] The most preferred methods for TBI dosimetry are Thermoluminescent Dosimetry (TLD), optically stimulated luminescence dosimetry, and MOSFET. Especially in phantom studies, TLD is chosen more than others.[11]
In this study, it was aimed to investigate TBI planned target volume (PTV) coverage and organ doses using helical tomotherapy. PTV dose and critical organ doses such as lens, lungs, and kidneys were measured by TLD on the phantom. PTV and critical organ doses on the treatment planning system (TPS) and measured TLD doses were compared.
Methods
In this study, first, radiation absorption of rice and water was compared. The same size plexiglass boxes were filled with rice and water. And then, CT images of water and rice were taken. The same beam was applied to them, and it was seen that they have the same absorption of the beam which means that rice can be considered as tissue equivalent material in radiotherapy. Figure 1 shows the CT procedure of water and rice, and isodoses on TPS.The arms and legs created using rice were added to the male phantom (CIRS, Computerized Imaging Reference Systems Inc. Virginia, USA) to simulate the whole body geometrically. The male phantom with arms and legs was immobilized by a vacuum bed. The CT images of the lower and upper region of the phantom with a slice thickness of 3 mm were acquired using Philips Big Bore CT (Philips Medical Systems, Cleveland, OH, USA). The CT datasets were transferred to the TPS. Figure 2 shows the male phantom with added rice arms and legs immobilized by a vacuum bed for taking CT images.
In Volo TPS (Accuray, Incorporated, Sunnyvale, CA), PTV was created as the entire body with 5 mm inner margin from the skin, and then, lungs, lens, and kidneys were contoured with a margin of 3 mm from the PTV. Dose constraints for organs at TPS were; mean coverage of 90% PTV is 12 Gy, maximum lens doses are 5 Gy, mean lung doses are 8 Gy, and mean kidney doses are 7 Gy. As planning parameters, 5.054 cm field width, 2.0 modulation factor, 0.415 pitch value, dynamic jaw mode, and helical beam mode were prescribed to TPS. The upper and lower part of the body was planned separately and united in MIM software (MIM Software, Cleveland, OH) to control hot and cold spots in intersecting areas.
45 number of GR-200A (PTW, Physikalisch Technische Werkstätten, Freiburg, Germany) with a diameter of 4 mm and 0.8 mm-thickness disc TLD were used. In this study, three groups of TLD were used. The standard deviations for each group of TLD were <2%. Each group of TLDs was calibrated to 1 Gy in a solid water equivalent phantom with a 10×10 cm2 field size at a depth of 5 cm and a source-axis distance is 100 cm. In the first part of the study, TLDs were placed into the male phantom and irradiated using TomoTherapy-HDA Treatment System (Accuray, Incorporated, Sunnyvale, CA) with helical tomotherapy technique. TLDs were read out with Fimel LTM Reader. Entrance-exit, midpoint doses of lens, lungs, and kidneys were measured. This procedure was repeated 2 times and average values were considered. The second part of the study, it was aimed to make a comparison of planned and measured doses. The TPS and TLD doses were evaluated by Wilcoxon test.
Results
TPS ProceduresIn this research, the body is separated into two parts lower and upper. Two plans were prepared, and then, overlapped areas were checked. According to dose constraints prescribed to the TPS, homogenous dose distributions were gained. Figure 3 shows the upper body dose volume histogram (DVH).
Fig. 3. The upper body dose volume histogram.
Organ doses for upper body plan at VoLo TPS are; PTVmax=14.45 Gy, right lungaverage=7.61 Gy, left lungaverage =7.70 Gy, right kidneyaverage=6.70 Gy, left kidneyaverage =6.51 Gy, right lensmax=2.94 Gy, left lensmax=3.18 Gy, and externalmax=14.45 Gy.
For the lower part of the body, Figure 4 shows corresponding DVH.
Lower part doses are; PTVmax=13.12 Gy and external max=13.12 Gy.
Fig. 4. DVH for the lower part of the body.
DVH: Dose volume histogram.
After evaluation of upper and lower body plans, two plans were brought together in MIM software program to check hot or cold spots at intersecting areas. Figure 5 shows unified dose distribution of lower and upper halves of the body. As shown in Figure 5, no hot or cold points were observed. Figure 6 shows DVH for unified plans.
Fig. 5. Unified dose distribution of the lower and upper halves of the body.
Fig. 6. DVH for unified plans.
DVH: Dose volume histogram.
All planning values were in desirable range for TBI treatments. To ensure this, dose homogeneity index (HI) was calculated with the shown formula. According to ICRU 83 HI close to zero means, PTV dose distribution is homogeneous.[12]
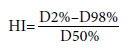
Calculated HI values in this study were for the upper body; 0.16, and for the lower body; 0.04. Results show that in this study, TPS provides homogenous dose distribution for PTV in TBI treatments.
In TBI treatments, since the target body is very large, time is another important factor for both patients and treatment team. To decrease, treatment time makes the process more comfortable and repeatable. One more advantage of tomotherapy is that it is very effective in decreasing treatment time in TBI treatments. In this study, treatment time for the upper body was 12.9 min, and for the lower body was 13.5 min. These numbers are very ideal for TBIs.
TPS Reading Doses and TLD Measured Doses
Comparison
After getting TPS values, 15 numbers of TLDs were
placed onto the male phantom to check TPS doses and
measured doses. TLDs were placed on the lens, head
midpoint, thyroid entrance-exit points, lungs midpoints,
between two lungs, kidneys midpoints, kidneys
entrance-exist points, two plan intersecting areas, and
between two feet. TLDs were placed at these points and irradiated 2 times. Average of two irradiation values
was calculated and made a comparison between
planned and measured doses of organs for one fraction.
The comparison was made with Wilcoxon test
and as shown in Table 1, there is no meaningful difference
between TPS and TLD doses (p>0.05) which
mean measured and planned doses are coherent.
Discussion
TBI is a type of radiotherapy that is applied with chemotherapy as a preparative regime for bone marrow trans- plantation and stem cell transplantation. The main purposes of TBI are to eliminate malignant cells, prevent graft rejections, and become ready for transplantation.[1,2]In TBIs, it becomes hard getting homogenous dose distribution due to inhomogeneous body shape and tissue density variations. The main goal of radiotherapy is to save critical organs while achieving a homogeneous dose distribution at the target volume. When it comes to deciding for TBI treatment method, some other crucial points must be considered. These considerations are treatment time, planning time, repeatability, and comfortableness for both patient and team.[2] When all these considerations are taken into account, tomotherapy stands out as the most ideal treatment technic for TBIs. The aim of this study is to investigate the suitability of tomotherapy for TBI and the coherence of planned and given doses.
For TBI treatments, 80% of the given dose are acceptable as the lungs" tolerance dose. 7-10 Gy lung mean dose is admissible and the midpoint of the lungs is seen as the reference point. Lens dose is admissible under 5 Gy for all treatments. These are dose limits for TBI in practice and measured doses in this study are in the desired range of limits. Tomotherapy is very effective for saving critical organs while achieving homogeneous dose distribution in PTV. The skin dose in TBI should not be under 90% of the given dose[12] and if the skin cannot get aimed dose; then, a bolus is needed. In this study, 2 Gy was given per fraction and it was seen that measured that the skin doses were not under 90% of given dose. This again shows ideality of tomotherapy for TBI.
Several publications demonstrated the practicality of tomotherapy for TBI. Penagaricano et al. made a study with four AML patients to investigate clinical feasibility of helical tomotherapy for TBI. TBI prescription was set that 80% of the clinical target volume received 12 Gy in six fractions, at two fractions per day. 3 mm CT images were used for plan and lungs and kidneys were saved as critical organs. 5 cm jaw width, 2.0 pitch value, and 0.287 modulation factor were used for the plan parameters. Planning average doses of OAR were set not to exceed 8 Gy and 10 Gy to the lungs and kidneys, respectively.
In the treatment process planned and delivered CTV doses ranged from 12.1 to 12.4 Gy and 11.9- 12.3 Gy, average planned and delivered lung doses ranged from 6.6 to 7.4 Gy and 6.5 to 7.4 Gy, respectively. Average planned and delivered left kidney doses ranged from 7.4 to 8.7 Gy and 7.2 to 8.6 Gy, respectively. Average planned and delivered right kidney doses ranged from 7.5 to 8.6Gy and 7.3 to 8.5 Gy. The planned and delivered dose HI ranged from 0.91 to 0.94 and from 0.90 to 0.95. All results convince feasibility of tomotherapy for TBI.[13]
Gruen et al.[14] conducted a study with 10 AML and ALL patients in the 4-22 years old range. Patients treated 12 Gy/6 fr, two fractions per day dose schema. For immobilization of patients, vacuum bed and head mask were used. The plan was prepared with the prescription of 95% of PTV get 12 Gy, average of lungs" doses set to not exceed 10 Gy. PTV doses for 10 patients ranged from 11.6 to 12.5 Gy, left lung average doses ranged from 7.72 to 10.36 Gy, and right lung average doses ranged from 7.55 to 10.12 Gy. Treatment times of patients in the range of 13.6 to 20.6 min.
Hui et al.[15] conducted a rando phantom study by prescribing 95% of PTV 13.2 Gy/ 8 fr dose with helical tomotherapy technique. In the study, plan parameters were 2.0 modulation factor, 5 cm jaw width, and 0.460 pitch value. As critical organs lungs, kidneys, eyes, heart, and liver were saved. TLD 100 was used to measure organ doses. Planned average organ doses in this study were PTV 14.78 Gy, lungs 8.6 Gy, eyes 3.19 Gy, kidneys 6.33 Gy, liver 6.19 Gy, heart 5.32 Gy, and HI 0.94. This study shows the user-friendliness of tomotherapy for TBI since appliers can save critical organs without extra shielding blocks or compensators.
In another study conducted by Zhuang et al.,[16] it was seen that tomotherapy is more effective than the long SSD technique in TBI treatments. Four patients were treated with both helical tomotherapy and SSD techniques by receiving 12 Gy/10 fr and made a comparison between treatments. In tomotherapy treatments, 5 cm jaw width, 0.4 pitch value, and 2.0 modulation factor were used as plan parameters and 90% of PTV received 12 Gy. In SSD treatments, SSD~400 cm, 160×160 cm2 field width, and 45°gantry were used as plan parameters and lung shielding block and compensator were used. Received helical tomotherapy and Linac doses were D90; 12.31±0.09 and 10.26±0.23, D1; 13.6±0.14 and 14.38±0.2, HI; and 1.10±0.001 and 1.39±0.001, respectively. Right lung doses; 5.40±0.12 and 8.95±0.31 and left lung doses; 5.44±0.13 and 8.34±0.12, respectively.
Sarradin et al. conducted a study with 11 patients who were treated between August 2014 and January 2016. The total dose was 12 Gy in six fractions in 3 days. The median age was 31 years, range from 18 to 57 years. The median D98% of PTV was 11.5 Gy, ranging from 6.6 to 11.9 Gy. The average of the mean dose to the lungs was 8.7 Gy, ranging from 8.5 to 9.3 Gy. The mean dose for the junction area was 12 Gy, ranging from 11.9 to 12.1 Gy. In the study, they saw that no patient had radiation pneumonitis. According to this study, Sarradin et al. affirmed the efficiency of helical tomotherapy in TBI treatments in terms of sparing organs at risk while achieving uniform dose coverage at target volume.[17]
Sun et al.[18] conducted a study with TBI patients at their institution from February 2012 to May 2013. 2 Gy was given in a single fraction. PTV was divided in two due to the length of the table. To perform delivery quality assurance cylindrical phantom, ionization chamber and films were used. Thermoluminescent dosimeters and radiochromic films were used for in vivo dosimetry and junction region heterogeneity assessment. Their results are as follows: planned V95% was covered by D95% and V2% did not exceed D107% for five of the six patients. The mean relative difference between measured and calculated absolute dose of the delivery quality assurance was always <2.5% (mean value±SD: 1%±0.67%). The difference between in vivo measured and the calculated dose was above 5% for only two out of 15 points (maximum: 10.2%, mean: 0.73±4.6%). Junction region heterogeneity was in average 5.8±1%. The total treatment session of TBI lasted 120 min, with a mean beam on time of 17.2±0.6 and 11.2±1.6 min for the upper and lower part of the body, respectively. According to their results, they confirmed that TBI using helical tomotherapy guaranteed high-dose homogeneity throughout the body and dose verification was achievable, showing a small difference between planned and delivered doses.
Inhomogeneous body shape and tissue density variations make it hard to achieve uniform dose distributions in TBI treatments. The most crucial consideration of radiotherapy is saving critical organs meanwhile achieving homogeneous dose distribution at the target volume. Demonstrated studies show that tomotherapy is a very effective method in TBI treatments since it achieves the main goal of radiotherapy and it is comfortable for both patient and user.
Conclusion
It was seen that while irradiating the whole body, critical organs can be saved and got homogenous dose distribution. On the other hand, TPS and TLD doses were very close to each other, and this shows the accuracy of the TPS of tomotherapy. It was also shown that TLD can be used to control treatments plan for TBI treatments.Peer-review: Externally peer-reviewed.
Conflict of Interest: All authors declared no conflict of interest.
Ethics Committee Approval: The study was approved by the Istanbul University, Institute of Oncology Ethics Committee (no: 338172, date: 22/09/2016).
Financial Support: None declared.
Authorship contributions: Concept - H.B.B., G.U.A.; Design - H.B.B., G.U.A., İ.K.Ç.; Supervision - H.B.B., G.U.A., C.K.A.; Funding - H.B.B., G.U.A., İ.K.Ç.; Materials - H.B.B., G.U.A.; Data collection and/or processing - H.B.B., G.U.A., İ.K.Ç.; Data analysis and/or interpretation - H.B.B., G.U.A., C.K.A.; Literature search - G.U.A., C.K.A.; Writing - H.B.B., G.U.A., İ.K.Ç.; Critical review - H.B.B., G.U.A., C.K.A., İ.K.Ç.
References
1) Wong JY, Liu A, Schultheiss T, Popplewell L, Stein A,
Rosenthal J, et al. Targeted total marrow irradiation
using three-dimensional image-guided tomographic
intensity-modulated radiation therapy: an alternative
to standard total body irradiation. Biol Blood Marrow
Transplant 2006;12(3):306-15.
2) Özdemir Ö, Hoca S, Olacak N, Özkök S, Kamer S,
Anacak Y, et al. Tüm vücut işınlamalarında orta hat
dozlarının belirlenmesi ve hesaplanan dozların doğrulanması.
Türk Onk Dergisi 2013;28(2);67-74.
3) Piotrowski T. Skorska M. Tomothrapy -a different way
of dose delivery in radiotherapy. Wspolczesna Onkol
2012;16(1):16-25
4) Mackie TR, Balog J, Ruchala K, Shepard D, Aldridge
S, Fitchard E, et al. Tomotherapy. Semin Radiat Oncol
1999;9(1):108-17.
5) Beavis AW. Is tomotherapy the future of IMRT? Br J
Radiol 2004;77(916):285-95.
6) Ekici K, Temelli Ö. Helical tomotherapy and its benefits.
J Turgut Ozal Med Cent 2014;21(4):321-2.
7) Seung SK, Larson DA, Galvin JM, Mehta MP, Potters L,
Schultz CJ, et al. American College of Radiology (ACR)
and American Society for Radiation Oncology (ASTRO)
practice guideline for the performance of stereotactic radiosurgery
(SRS). Am J Clin Oncol 2013;36(3):310-5.
8) Storb R, Raff RF, Appelbaum FR, Deeg HJ, Graham
TC, Schuening FG, et al. Fractionated versus singledose
total body irradiation at low and high dose rates
to condition canine littermates for DLA-identical marrow
grafts. Blood 1994;83(11):3384-9.
9) Storb R, Raff RF, Appelbaum FR, Graham TC, Schuening
FG, Sale G, et al. Comparison of fractionated to
single-dose total body irradiation in conditioning
canine littermates for DLA-identical marrow grafts.
Blood 1989;74(3):1139-43.
10) Mulcahy Levy JM, Tello T, Giller R, Wilkening G,
Quinones R, Keating AK, et al. Late effects of total
body irradiation and hematopoietic stem cell transplant
in children under 3 years of age. Pediatr Blood
Cancer 2013;60(4):700-4.
11) Briot E, Dutreix A, Bridier A. Dosimetry for total body
irradiation. Radiother Oncol 1990;18(Suppl 1):16-29.
12) ICRU Report No 83. Prescribing, Recording, and Reporting
Photon-Beam Intensity-Modulated Radiation
Therapy (IMRT). Strahlenther Onkol 2010;10(1).97-9.
13) Peñagarícano JA, Chao M, Van Rhee F, Moros EG,
Corry PM, Ratanatharathorn V. Clinical feasibility of
TBI with helical tomotherapy. Bone Marrow Transplant
2011;46(7):929-35.
14) Gruen A, Ebell W, Wlodarczyk W, Neumann O, Kuehl
JS, Stromberger C, et al. Total Body Irradiation (TBI)
using Helical Tomotherapy in children and young
adults undergoing stem cell transplantation. Radiat
Oncol 2013;8:92.
15) Hui SK, Kapatoes J, Fowler J, Henderson D, Olivera G,
Manon RR, et al. Feasibility study of helical tomotherapy
for total body or total marrow irradiation. Med
Phys 2005;32(10):3214-24.
16) Zhuang AH, Liu A, Schultheiss TE, Wong JY. Dosimetric
study and verification of total body irradiation
using helical tomotherapy and its comparison to extended
SSD technique. Med Dosim 2010;35(4):243-9.





