2Department of Medical Sciences, Shahroud University of Medical Sciences, Faculty of Paramedical, Shahroud-Iran
3Student Research Committee, Shahroud University of Medical Sciences, Shahroud-Iran DOI : 10.5505/tjo.2022.3512
Summary
OBJECTIVEThe anticancer properties of curcumin were determined in several studies, but on breast cancer stem cells (BCSCs) derived from a heterogeneous population of tumor cells, these effects have not been previously reported. This study aimed to evaluate these effects.
METHODS
After the development of an animal model of breast cancer, a heterogeneous population of breast cancer
cells were isolated from the tumor mass. Spheroid formation as a reliable in vitro assay to assess the presence
of BCSCs was conducted among these cells. The cytotoxic activity of curcumin on multicellular
breast cancer spheroids was assessed by MTT assays. Induction of apoptosis was measured by Annexin
V-propidium iodide (pi) flow cytometric analysis.
RESULTS
The curcumin has potent cytotoxic and apoptotic effects on breast cancer spheroids. Although, compared
with monolayer, breast cancer cells are more resistant to apoptosis when cultured as multicellular
spheroids.
CONCLUSION
This is the first report of the anticancer effects of curcumin on breast cancer stem like cells. Compared
to many anti-cancer drugs and compounds, which have very limited ability to fight cancer stem cells,
curcumin is a good candidate to combat BCSCs.
Introduction
Despite many years of clinical research, statistics showed the number of diagnosed cases continues to rise and breast cancer remains the most common malignancy among ladies around the world.[1] The challenging characteristics of breast cancer such as metastasis, recurrence, and reduced overall survival are caused by breast cancer stem cells (BCSCs).[2] Accordingly, eradication of BCSCs is likely to be of clinical importance.[3] Multicellular tumor spheroids or in vitro three-dimensional (3D) culture systems are widely used models in tumor research.[4] Nowadays, multicellular tumor spheroids are the main candidate models in tumor research due to preserving the biological characteristics of original tumors better than conventional two-dimensional (2D) monolayer cultures. [5] These spheroids are purposed for the enrichment of cancer stem cells (CSCs) or cells with stem cell-related characteristics.[6]Herbal extracts and their active components are a promising candidate for new treatment strategies against a variety of diseases included malignancies. [7] Curcuma genus has been extensively used in traditional medicine for treating several diseases.[8] One of the constituents of Curcuma species is curcuminoids including curcumin.[9] Antioxidant, anti-inflammatory, antimicrobial, antiviral, and anticancer effects are the main biological activities of these phytochemicals. Among all, its anticancer potential has been the most described and remains under investigation.[10] Curcumin is believed to show its impact on cell growth and invasion of breast cancer through inducing apoptosis by regulating the expression of apoptosis-related genes, cell cycle arrest at the G2M phase and late S phase.[11-13] Studies have demonstrated that curcumin alone or combined with other drugs presents anticancer activities against malignant cell lines.[14]
Several studies have been focused on anticancer activities of curcumin but there is a lack of enough information on the effects of curcumin on multicellular breast cancer spheroids. In the present study, we are aimed to elucidate the anticancer properties such as cytotoxicity and apoptotic effects of curcumin on breast cancer multicellular tumor spheroids which are a great candidate on representing the native tumor microenvironment.
Methods
Cell CultureMurine 4T1 cell line was obtained from the cell bank of Pasteur Institute of Iran (C604). These breast cancer cells were cultivated in high glucose Dulbecco"s Modified Eagle's Medium (DMEM) in the presence of 10% fetal bovine serum (FBS) and 2% penicillin-streptomycin (all from Gibco, USA) in humidified atmosphere of 5% CO2 at 37ºC.
Induction of Syngeneic Animal Model of Breast Cancer
As described in previous works[15] for tumor induction
in female BALB/c mice, 4T1 cells were subcutaneously
injected to the flank (or the right hind limb)
of the mice (105 cells suspended in 100 µL phosphatebuffered
saline [PBS]) using an insulin syringe. All animal
experiments were in compliance with the relevant
laws, and this study was approved by the Ethics Committee
of Shahrood University of Medical Sciences
(registration number: IR.SHMU.REC.1398.109).
Preparation of Heterogeneous Population of Breast
Cancer Cells
CSCs are a small subset of the cancer cells among a
heterogeneous population of cancer cells. Accordingly,
for the first step, it is necessary to dissociate
a tumor tissue sample into a single cell suspension
to be able to isolate CSCs from the rest of the cancer
cells. In the present research for isolation of heterogeneous
population of tumor cells, primary tumor of
cancerous mice was excised after 20 days of tumor
induction in mice, and surface blood was removed by
rinsing it in PBS. After mincing with scissors, fragments
were placed to 50 ml conical tube. For enzymatic
digestion, primary tumor was digested in 10
mg/ml collagenase type IV at 37°C for 75 min on a
platform rocker. All enzymes were purchased from
Sigma (St. Louis, MO, USA). The digested tumor filtered
through 70 um cell strainers and washed with
PBS. In the next step, washed cells were resuspended
in medium containing 10% FBS, 100 U/ml penicillin,
and 100 ug/ml streptomycin (all from Gibco, USA).
Ultimately, the cells were cultured at 37°C in 5% CO2
and passaged 2 times.
Cytotoxic Effect of Curcumin on Breast Cancer
Spheroids
Suspension of heterogeneous population of cancer cells
(isolated in previous steps) was prepared and cultured
as described below:
1. For 2D monolayer, cells were seeded at a density of 1×104 cells/well in a 96-well culture plates and cultured in high glucose DMEM containing 10% FBS and 2% penicillin-streptomycin (all from Gibco, USA) in humidified atmosphere of 5% CO2 at 37ºC. After 24 h incubation, cell culture medium was exchanged with complete medium supplemented with different concentrations of curcumin (5, 10, 15, 20, and 30 µM). Following 48 h incubation at 37ºC, medium was removed and 50 ml of MTT solution at 5 mg/ml (Sigma) was added to the cultures and the incubation continued for a further 4 h period, after which 150 ml of dimethyl sulfoxide (DMSO) was added. Formed formazan crystals were allowed to dissolve for 30 min before measuring the optical density at 570 nm using CYTATION/5 imaging reader (Bio-Tek Instrument, USA). Finally, cell viability was expressed as percent compared to control wells according to the following equation:
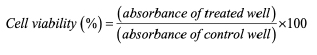
In this equation, blank means culture medium without cells and control means culture medium with cells. This experiment was performed in triplicate.
2. To form 3D spheroids, cells were seeded at a density of 1×104 cells/well in a 96-well Ultra-Low Attachment microplate and cultured in spheroid forming media comprised high glucose DMEM containing 0.5% FBS and 2% penicillin-streptomycin (all from Gibco, USA) in humidified atmosphere of 5% CO2 at 37°C and incubating them for 6 days. The resulting tumor spheroids were treated for 48 h with different concentrations of curcumin (10, 25, 50, 75, and 100 µM) and MTT assay was performed as explained above.
Apoptosis Assay
For apoptosis assay, similar to previous step, 2D monolayer
and 3D spheroids of breast cancer cells were prepared
and treated for 48 h with IC50 concentrations of
curcumin. Cell apoptosis assays were carried out with
the use of MabTag's Annexin-V Apoptosis Detection
Kit, according to the manufacturer protocol.
Statistical Analysis
Results are expressed as the mean ± standard deviation.
Data were analyzed with GraphPad Prism statistical
software 6.0 (GraphPad Software, La Jolla, CA, USA)
using paired samples t-test. P<0.05 was considered statistically
significant.
Results
Isolation of Heterogeneous Population of Tumor CellsMost breast cancer cell lines have undergone changes in their function and genome due to multiple passages and manipulations.[16] Hence, we decided to use the heterogeneous population of primary tumor cells to create spheroid. For this, animal model of triple-negative breast cancer was generated (Fig. 1a). When tumor mass is palpable (20 days following tumor induction in BALB/c mice), tumor mass was removed aseptically (Fig. 1b). H and E staining and pathological confirmation were performed on tumor tissues (Fig. 1b). Heterogeneous population of tumor cells was isolated from tumor mass with enzymatical and mechanical digestion (Fig. 1c).
Multicellular Breast Cancer Spheroids Formation
For spheroid formation among heterogeneous population
of tumor cells, we used non-adherent 96 well
plates. As shown in Figure 1, after 6 days, the spheroids
formed in the well (Fig. 1d). At this stage, the spheroids
were ready for treatment with the curcumin.
Cytotoxic Effects of Curcumin against Multicellular
Breast Cancer Spheroids
To determine the growth inhibitory activity of curcumin
on breast cancer cells, heterogeneous population
of tumor cells were treated with different concentrations
of curcumin for 24 h, 48 h, and 72 h and cells
viability was measured by MTT assay. The initial results
indicated that cytotoxic effect was better analyzable
qualitatively and statistically after 48 h. Therefore, the
MTT assay was only done in dose-dependent manner.
Exposing heterogeneous population of primary tumor
cells to curcumin resulted in a significant decrease in
cells viability in a dose-dependent manner (p<0.05),
(Fig. 2). The IC50 value was considered as the concentration
of the curcumin that caused a 50% decrease in
cell viability relative to the negative control which was
constituted by cell culture and DMSO without the curcumin.
For 2D monolayer of tumor cells, IC50 value
was found to be 10 uM by MTT assay. Compared with
2D monolayer, breast cancer cells are more resistant to
cytotoxic effects of curcumin when cultured as multicellular 3D spheroids. IC50 value was found to be 60
uM by MTT assay for tumor cells presented in multicellular
3D spheroids. These IC50 concentrations were
selected for all further mechanistic studies.
Apoptotic Effects of Curcumin
To determine the apoptotic effects of the curcumin, we
used annexin test. For this purpose, both the heterogeneous
population of tumor cells in 2D conditions and
the population of cancer stem cells located in spheroids
were treated with a concentration of IC50 concentrations
of curcumin. The results of Annexin V/PI staining
for curcumin after 48 h are shown in Figure 3. The
curcumin could be induced apoptosis in breast cancer
cells both in 2D and 3D condition but result indicated
that breast tumor cells are significantly more resistant
to apoptosis in 3D multicellular spheroids.
Discussion
Results of this study demonstrate that curcumin has apoptotic and cytotoxic effects against multicellular breast cancer spheroids. After preparation and isolation of a heterogeneous population of tumor cells, spheroid formation was conducted among these cells. The MTT assay results showed that in a dose-dependent manner, there was a significant decrease in cells viability. It should be noted that the cytotoxic effects of the curcumin on the spheroids were significantly reduced compared to 2D culture conditions. The apoptotic effects of curcumin were evaluated by the annexin test which showed apoptosis induction in both 2D conditions and the population of cancer stem cells located in spheroids, but the level of apoptosis was significantly lower in spheroids.Curcumin showed cytotoxic and apoptotic potential against breast cancer in other studies as well. Several mechanisms such as inhibition of oncogene protein expression, stem-like properties, regulating the EMT process, cell cycle arrest, and interaction with oncogenic and tumor-suppressive miRNAs underlie curcumin cytotoxic effects.[13,17-19]
Most cytotoxicity assays are designed to evaluate anticancer drug effects on classic 2D cultures. Consequently, many cell characteristics and dynamic nature of tumor microenvironments are usually lost so 3D models are more predictive than monolayers in 2D cultures.[20,21] Similar to our findings, Abuelba et al.[22] also reported slight cytotoxic reduction in 3D breast cancer model compared to 2D condition. They worked on metastatic breast adenocarcinoma cell line (MDA-MB-231) and concluded that 3D tumor cell culture systems appear to be the ideal environment for in vitro assays regarding anticancer drug effects on cell viability.
Most 3D culture models are supposing tumor cell seeding on polymer scaffolds or cell embedding in hydrogels while some models are speculating adherent cell ability to cluster in suspension or on low adherence surface. In a recent work, for achieving a 3D environment, researcher used encapsulation of cells in alginate hydrogel. The results of this study showed that curcumin in 3D culture conditions causes mortality in breast cancer cells (MCF-7).[23] Because of cell behavior in cell clusters is essential to further development of the tumor microenvironment, in our work, we used spheroids for 3D culture of breast cancer cells.
Evaluation of human MCF-7 breast cancer cells seeded in 2 and 3D culture systems confirmed that curcumin significantly decreased MCF-7 cells viability in dose? and time?dependent manners in 2 and 3D systems. However, cell viability in 2D cultures was significantly lower compared to 3D cultures; most probably due to cell clustering effect that may prevent curcumin penetration to inner cells in spheroids.[24] In this work, researchers used human MCF-7 breast cancer cell line. Most breast cancer cell lines have undergone changes in their function and genome due to multiple passages and manipulations.[16] Accordingly, in our work, we use the heterogeneous population of primary tumor cells to create spheroid.
It should be noted that curcumin effectiveness has been limited due to low bioavailability.[25] Several studies have been conducted to increase curcumin effectiveness.[26] To boost the bioavailability of this chemical component, various approaches have been undertaken like using adjuvant, liposomal curcumin, nanoparticles, phospholipid complex, and structural analogs of curcumin.[25]
In case of clinical use, since cancer is still one of the leading causes of death in the world, there is an increasing demand of new therapeutic interventions. [27] According to Sen et al.[28] study, curcumin is a potent chemosensitizer that improves the therapeutic index of widely used anti-cancer drugs. Therefore, it can be developed into an adjuvant chemotherapeutic drug. Xiong et al.[29] designed a dual-drug codelivery system which consisted of PTX (a US Food and Drug Administration approved chemotherapeutic drug for the treatment of breast cancer) and curcumin. The results of this in vivo study suggest that dual drugs could be a potential system for the treatment of breast cancers. Ferguson and Orlando also reported that addition of curcumin during 5-fluorouracil (an antimetabolite that inhibits cell proliferation) therapy enhanced the chemotherapeutic effectiveness by protecting normal cells from reduced viability and consequently permitting higher dosing or longer treatment times.[30]
Conclusion
The outcomes of this study clearly show that curcumin is capable of inducing apoptosis and has cytotoxic effects on both 2D and 3D breast cancer models. Up-todate findings on curcumin anti-cancer properties suggest that it could open a new era in cancer treatment but further investigations are obligate.[31]Acknowledgments: We would like to thank the research assistant of Shahrood University of Medical Sciences and all the participants who helped us in this project.
Peer-review: Externally peer-reviewed.
Conflict of Interest: The author declares that they have no competing interest.
Ethics Committee Approval: This study was approved by the Shahrood University of Medical Sciences Ethics Committee (No: IR.SHMU.REC.1400.027, Date: 08/05/2021).
Financial Support: This work was supported by a grant from the Shahrood University of Medical Sciences (SHMU) grant no. 99130.
Authorship contributions: Concept - M.K.F.; Design - M.K.F.; Supervision - M.K.F.; Funding - M.K.F., A.Atashi; Materials - M.K.F., A.Atashi; Data collection and/or processing - M.K.F., A.Asadi; Data analysis and/or interpretation - M.K.F., A.Atashi; Literature search - M.K.F., A.Asadi; Writing - M.K.F., A.Asadi; Critical review - M.K.F., A.Atashi
References
1) Britt KL, Cuzick J, Phillips K-A. Key steps for effective
breast cancer prevention. Nat Rev Cancer
2020;20(8):417-36.
2) Butti R, Gunasekaran VP, Kumar TVS, Banerjee P,
Kundu GC. Breast cancer stem cells: Biology and
therapeutic implications. Int J Biochem Cell Biol
2019;107:38-52.
3) Bai X, Ni J, Beretov J, Graham P, Li Y. Cancer stem cell
in breast cancer therapeutic resistance. Cancer Treat
Rev 2018;69:152-63.
4) Ishiguro T, Ohata H, Sato A, Yamawaki K, Enomoto
T, Okamoto K. Tumor-derived spheroids: Relevance to
cancer stem cells and clinical applications. Cancer Sci
2017;108(3):283-9.
5) Nath S, Devi GR. Three-dimensional culture systems
in cancer research: Focus on tumor spheroid model.
Pharmacol Ther 2016;163:94-108.
6) Froehlich K, Haeger JD, Heger J, Pastuschek J, Photini
SM, Yan Y, et al. Generation of multicellular breast
cancer tumor spheroids: comparison of different protocols.
J Mammary Gland Biol Neoplasia 2016;21(3-4):89-98.
7) Yeung KS, Gubili J, Mao JJ. Herb-drug interactions
in cancer care. Oncology (Williston Park)
2018;32(10):516-20.
8) Rahaman MM, Rakib A, Mitra S, Tareq AM, Emran
TB, Shahid-Ud-Daula AFM, et al. The genus curcuma
and inflammation: overview of the pharmacological
perspectives. Plants (Basel) 2020;10(1):63.
9) Sharma RA, Gescher AJ, Steward WP. Curcumin: the
story so far. Eur J Cancer 2005;41(13):1955-68.
10) Giordano A, Tommonaro G. Curcumin and cancer.
Nutrients 2019;11(10):2376.
11) Ramachandran C, Fonseca HB, Jhabvala P, Escalon
EA, Melnick SJ. Curcumin inhibits telomerase activity
through human telomerase reverse transcritpase
in MCF-7 breast cancer cell line. Cancer Lett
2002;184(1):1-6.
12) Lv ZD, Liu XP, Zhao WJ, Dong Q, Li FN, Wang HB, et
al. Curcumin induces apoptosis in breast cancer cells
and inhibits tumor growth in vitro and in vivo. Int J
Clin Exp Pathol 2014;7(6):2818-24.
13) Liu D, Chen Z. The effect of curcumin on breast cancer
cells. J Breast Cancer 2013;16(2):133-7.
14) Mehta K, Pantazis P, McQueen T, Aggarwal BB. Antiproliferative
effect of curcumin (diferuloylmethane)
against human breast tumor cell lines. Anticancer
Drugs 1997;8(5):470-81.
15) Farahani MK. High capacity of the metastatic breast
tumor cells in sphere formation: clue for chemoresistance
in triple-negative breast cancer. Turk J Oncol
2020;35(4):466-70.
16) MacLeod RA, Dirks WG, Matsuo Y, Kaufmann M,
Milch H, Drexler HG. Widespread intraspecies crosscontamination
of human tumor cell lines arising at
source. Int J Cancer 1999;83(4):555-63.
17) Hu C, Li M, Guo T, Wang S, Huang W, Yang K, et al.
Anti-metastasis activity of curcumin against breast
cancer via the inhibition of stem cell-like properties
and EMT. Phytomedicine 2019;58:152740.
18) Hasan M, Belhaj N, Benachour H, Barberi-Heyob M,
Kahn CJ, Jabbari E, et al. Liposome encapsulation of
curcumin: physico-chemical characterizations and effects
on MCF7 cancer cell proliferation. Int J Pharm
2014;461(1?2):519-28.
19) Norouzi S, Majeed M, Pirro M, Generali D, Sahebkar
A. Curcumin as an adjunct therapy and microRNA
modulator in breast cancer. Curr Pharm Des
2018;24(2):171-7.
20) Imamura Y, Mukohara T, Shimono Y, Funakoshi Y,
Chayahara N, Toyoda M, et al. Comparison of 2D- and
3D-culture models as drug-testing platforms in breast
cancer. Oncol Rep 2015;33(4):1837-43.
21) Prince E, Kheiri S, Wang Y, Xu F, Cruickshank J,
Topolskaia V, et al. Microfluidic arrays of breast tumor
spheroids for drug screening and personalized cancer
therapies. Adv Healthc Mater 2022;11(1):e2101085.
22) Abuelba H, Cotrutz CE, Stoica BA, Stoica L, Olinici D,
Petreuş T. In vitro evaluation of curcumin effects on
breast adenocarcinoma 2D and 3D cell cultures. Rom
J Morphol Embryol 2015;56(1):71-6.
23) Zargan S, Salehi Barough M, Zargan J, Shayesteh M,
Haji Noor Mohammadi A, Mousavi M, et al. Evaluation
of the anti-cancer effect of curcumin on MCF-7
cells in 3D culture conditions to increase the efficacy
of breast cancer treatment. J Appl Biotechnol Rep
2022;9(1):547-56.
24) El Feky SE, Ghany Megahed MA, Abd El Moneim
NA, Zaher ER, Khamis SA, Ali LMA. Cytotoxic,
chemosensitizing and radiosensitizing effects of curcumin
based on thioredoxin system inhibition in
breast cancer cells: 2D vs. 3D cell culture system. Exp
Ther Med 2021;21(5):506.
25) Anand P, Kunnumakkara AB, Newman RA, Aggarwal
BB. Bioavailability of curcumin: problems and promises.
Mol Pharm 2007;4(6):807-18.
26) Tajbakhsh A, Hasanzadeh M, Rezaee M, Khedri M,
Khazaei M, ShahidSales S, et al. Therapeutic potential
of novel formulated forms of curcumin in the treatment
of breast cancer by the targeting of cellular and
physiological dysregulated pathways. J Cell Physiol
2018;233(3):2183-92.
27) Zaimy MA, Saffarzadeh N, Mohammadi A,
Pourghadamyari H, Izadi P, Sarli A, et al. New methods
in the diagnosis of cancer and gene therapy of
cancer based on nanoparticles. Cancer Gene Ther
2017;24(6):233-43.
28) Sen GS, Mohanty S, Hossain DMS, Bhattacharyya
S, Banerjee S, Chakraborty J, et al. Curcumin enhances
the efficacy of chemotherapy by tailoring
p65NF?B-p300 cross-talk in favor of p53-p300 in
breast cancer. J Biol Chem 2011;286(49):42232-47.
29) Xiong K, Zhang Y, Wen Q, Luo J, Lu Y, Wu Z, et al. Codelivery
of paclitaxel and curcumin by biodegradable
polymeric nanoparticles for breast cancer chemotherapy.
Int J Pharm 2020;589:119875.





